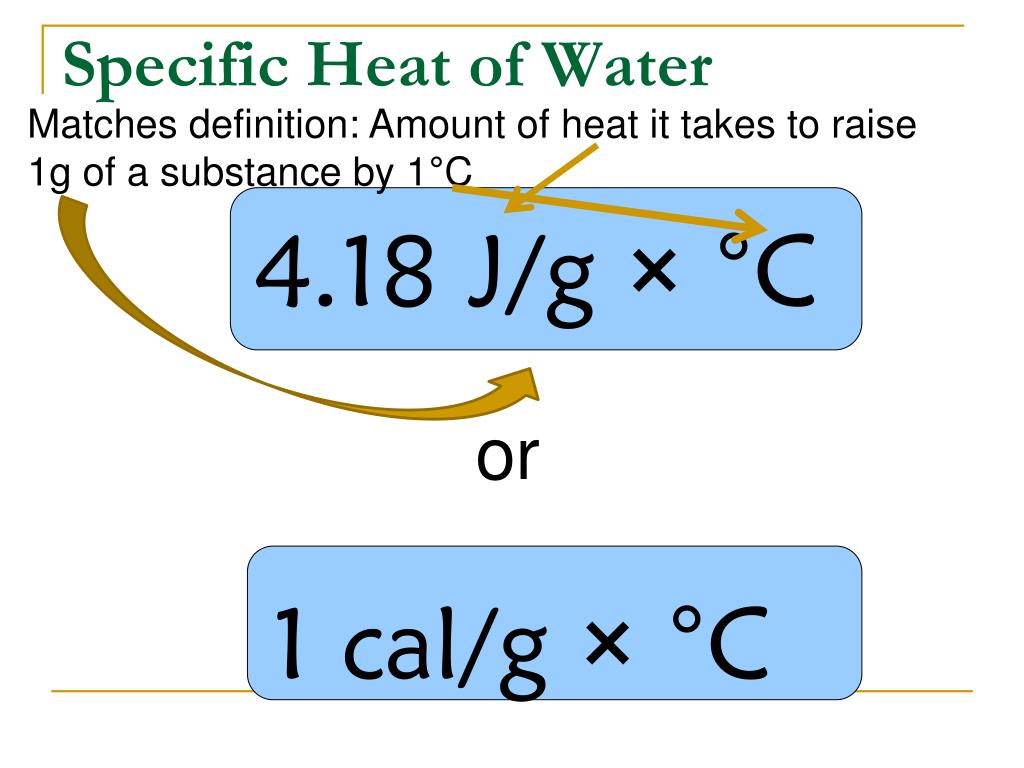
What Is Specific Heat Capacity Units . The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit. It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat capacity of a material is the amount of heat energy required to raise a unit mass of that material by 1 kelvin. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Specific heat capacity refers to the amount of heat or energy needed to raise the temperature of a substance with a fixed volume. It describes how much heat must be added to a.
from www.slideserve.com
Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. It describes how much heat must be added to a. It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. The specific heat capacity of a material is the amount of heat energy required to raise a unit mass of that material by 1 kelvin. Specific heat capacity refers to the amount of heat or energy needed to raise the temperature of a substance with a fixed volume.
PPT Unit 09 PowerPoint Presentation, free download ID5621270
What Is Specific Heat Capacity Units It describes how much heat must be added to a. The specific heat capacity of a material is the amount of heat energy required to raise a unit mass of that material by 1 kelvin. It describes how much heat must be added to a. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Specific heat capacity refers to the amount of heat or energy needed to raise the temperature of a substance with a fixed volume. It plays a crucial role in understanding. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit.
From www.tes.com
Specific heat capacity complete lesson (GCSE 19) Teaching Resources What Is Specific Heat Capacity Units Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit. Specific heat capacity refers to the amount of heat or energy needed to raise the temperature of a substance with a fixed volume. The specific heat capacity of a material is the amount. What Is Specific Heat Capacity Units.
From www.slideserve.com
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free What Is Specific Heat Capacity Units Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. The specific heat capacity of a material is the amount of heat energy required to raise a unit mass of that material by 1 kelvin. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance. What Is Specific Heat Capacity Units.
From askfilo.com
The differences between specific heat capacity and heat capacity are.. What Is Specific Heat Capacity Units It plays a crucial role in understanding. The specific heat capacity of a material is the amount of heat energy required to raise a unit mass of that material by 1 kelvin. Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit. The. What Is Specific Heat Capacity Units.
From goffheatingandairspringfieldmowaritsu.blogspot.com
Goff Heating And Air Springfield Mo Specific Heat Units What Is Specific Heat Capacity Units Specific heat capacity refers to the amount of heat or energy needed to raise the temperature of a substance with a fixed volume. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It plays a crucial role in understanding. It describes how much heat. What Is Specific Heat Capacity Units.
From www.tessshebaylo.com
Equation For Energy Transferred Specific Heat Capacity Tessshebaylo What Is Specific Heat Capacity Units Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to. What Is Specific Heat Capacity Units.
From mytutorsource.hk
Learn About Specific Heat Capacity in Less Than 10 Minutes! What Is Specific Heat Capacity Units It describes how much heat must be added to a. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Specific heat capacity refers to the amount of heat or energy needed to raise the temperature of a substance with a fixed volume. Specific heat is defined as. What Is Specific Heat Capacity Units.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID831852 What Is Specific Heat Capacity Units It plays a crucial role in understanding. Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. The specific heat capacity of a material is the amount of. What Is Specific Heat Capacity Units.
From studylib.net
1.3 Specific Heat Capacity What Is Specific Heat Capacity Units Specific heat capacity refers to the amount of heat or energy needed to raise the temperature of a substance with a fixed volume. It describes how much heat must be added to a. It plays a crucial role in understanding. Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied. What Is Specific Heat Capacity Units.
From www.slideserve.com
PPT 4.3.3 Thermal properties of materials PowerPoint Presentation What Is Specific Heat Capacity Units Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit. The specific heat capacity of a material is the amount of heat energy required to raise a unit mass of that material by 1 kelvin. It describes how much heat must be added. What Is Specific Heat Capacity Units.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID3721637 What Is Specific Heat Capacity Units Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. It plays a crucial role in understanding. The specific heat capacity of a material is the amount of heat energy required to raise a unit mass of that material by 1 kelvin. The specific heat capacity is the amount of heat it takes to change. What Is Specific Heat Capacity Units.
From www.slideserve.com
PPT Unit 09 PowerPoint Presentation, free download ID5621270 What Is Specific Heat Capacity Units Specific heat capacity refers to the amount of heat or energy needed to raise the temperature of a substance with a fixed volume. The specific heat capacity of a material is the amount of heat energy required to raise a unit mass of that material by 1 kelvin. Heat capacity or thermal capacity is a physical property of matter, defined. What Is Specific Heat Capacity Units.
From brainly.in
difference between specific heat and heat capacity Brainly.in What Is Specific Heat Capacity Units Specific heat capacity refers to the amount of heat or energy needed to raise the temperature of a substance with a fixed volume. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by. What Is Specific Heat Capacity Units.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Specific Heat Capacity Units The specific heat capacity of a material is the amount of heat energy required to raise a unit mass of that material by 1 kelvin. Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit. It plays a crucial role in understanding. Specific. What Is Specific Heat Capacity Units.
From www.gkseries.com
SI unit of specific heat capacity is What Is Specific Heat Capacity Units It plays a crucial role in understanding. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit. Specific heat is defined as the amount of heat required to. What Is Specific Heat Capacity Units.
From caitlin-jolpbloghaney.blogspot.com
Specific Heat Capacity Units What Is Specific Heat Capacity Units Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. It describes how much heat must be added to a. Specific heat capacity refers to the amount of heat or energy needed to. What Is Specific Heat Capacity Units.
From thechemistrynotes.com
Heat Capacity and Specific Heat What Is Specific Heat Capacity Units The specific heat capacity of a material is the amount of heat energy required to raise a unit mass of that material by 1 kelvin. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied. What Is Specific Heat Capacity Units.
From youtube.com
Chemistry 10.2 Specific Heat Capacity YouTube What Is Specific Heat Capacity Units Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit. Specific heat capacity refers to the amount of. What Is Specific Heat Capacity Units.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Specific Heat Capacity Units It describes how much heat must be added to a. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat capacity of a material is the amount of heat energy required to raise a unit mass of that material by 1 kelvin.. What Is Specific Heat Capacity Units.